Q1. (a) Does the first law of thermodynamics violate the law of conservation of energy?
(b) Write the limitations of the first law of thermodynamics.
Solution
(a) No, it is in conformity with the law of conservation of energy.
(b) The limitations of the first law of thermodynamics are:
(i) There is no indication available as regards the direction in which the change takes place.
(ii) It does not give any idea about the extent to which the change takes place.
Q2. A piece of lead is hammered. Does its internal energy increase? Does the heat enter the lead from outside?
Solution
Yes, internal energy of lead increases. No heat energy from outside enters the lead.
Q3. Answer the following questions :
(i) A piece of iron is hammered. Does the internal energy of the iron increase? Does the heat enter the piece of the iron from outside?
(ii) A hot piece of lead is immersed in cold water. Has the internal energy of the water increased? Has any work been done by the lead?
(iii) Why are the brake-drums of a car heated when the car moves down a hill at constant speed?
(iv) Two balls of the same metal having masses 10g and 20g collide with a target with the same velocity. If the total energy is used in heating the balls, which ball will attain higher temperature?
(v) An ideal gas is compressed at a constant temperature. Will its internal energy increase or decrease?
(vi) How can the internal energy of an ideal gas be changed?
Solution
(i) Yes. the internal energy increases. No. the heat does not enter the piece of iron from outside.
(ii) Yes. the increase in the internal energy is due to the transfer of heat and not due to any work.
(iii) Since the speed of the car is not increasing, the gravitational potential energy is converted into internal energy of the system (brake-drums) which gets heated.
(iv) The rise in temperature will be same for both the balls.
(v) Because the internal energy of an ideal gas depends only temperature, the internal energy remains the same.
(vi) The internal energy of an ideal gas can altered by compressing or by adiabatic expansion.
Q4. (a) Write an adiabatic relation between pressure and volume.
(b) Write an adiabatic relation between:
(i) Volume and temperature
(ii) Pressure and temperature
Solution
a) PVγ = constant, where γ = CP/CV
(b) (i) TV(γ-1) = constant
(ii) 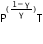 = constant
or P(1-γ)Tγ = constant
= constant
or P(1-γ)Tγ = constant
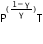 = constant
or P(1-γ)Tγ = constant
= constant
or P(1-γ)Tγ = constant
Q5. 200 joule of work is done on a gas to reduce its volume by compressing it. If this change is done under adiabatic conditions, find out the change in internal energy of the gas and also the amount of heat absorbed by the gas.
Solution
In adiabatic changes dQ = 0
Therefore, dQ = dU + dW = 0
dU = - dW = - ( - 200 J ) = 200J
Internal energy increases by 200J. Heat absorbed is zero.
Q6. An engine has been designed to work between source and sink at temperature 177ºC and 27ºC respectively. If energy input is 3600 J, what is the work done by the engine?
Solution
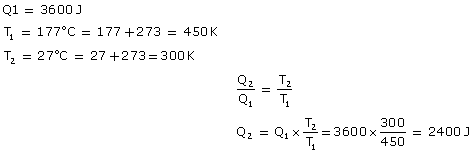
Q7. A carnot engine absorbs 1000J of heat energy from a reservoir at 12.7oC and rejects 600J of heat energy during each cycle. Calculate
(i) efficiency of the engine
(ii) temperature of sink
(iii) amount of useful work done per cycle.
Solution
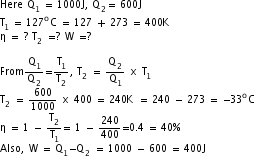
Q8. Refrigerator P works between - 10oC and 27oC, while refrigerator Q works between -27oC and 17oC. Both of them remove 2000 J heat from the freezer. Which of the two is a better refrigerator?
Solution
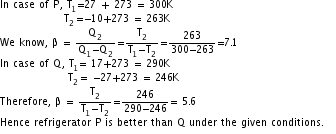
Q9. How does the Second Law of Thermodynamics help in the working of a refrigerator?
Solution
A way of stating the second law of thermodynamics is that work must be done to get heat to flow from a cold object to a hot object. In a refrigerator, there is a cycle that is carried on continuously. A liquid refrigerant substance vaporizes in the cooling coils inside the fridge. The fluid absorbs heat from its surroundings to vaporize. This cools the interior of the fridge. The gas thus formed is pumped to the exterior of the fridge where it is compressed into a liquid. Work is done on the gas to compress the gas, causing the gas to release heat. This heat is lost to the air surrounding the fridge. Hence, heat is moved from the inside of the fridge to the outside.
Q10. Explain the following terms in context with a heat engine:
(i) Hot reservoir (or source)
(ii) Cold reservoir (or sink)
(iii) Working substance
(iv) Mechanical parts
Solution
(i) Hot reservoir supplies heat energy which is to be converted into work.
(ii) Cold reservoir is that sub-system of the engine at lower temperature, where the part of the heat which has not been converted into work, is rejected.
(iii) Working substance is that part which receives some heat (Q1) from the hot substance (figure) and converts a part of it, i.e., (Q1-Q2) into work (W) and rejects the remaining Q2 to the surroundings.
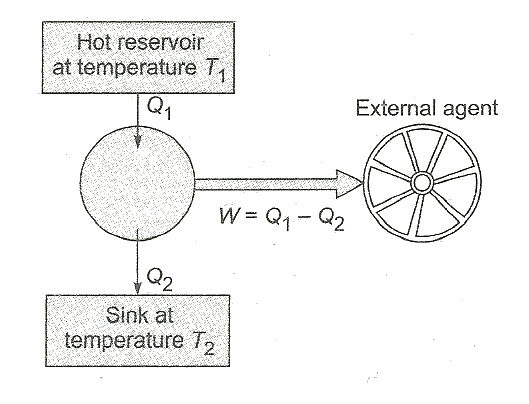 (iv) The agents which transfer the energy from the engine to the external agents are mechanical parts. The cylinder with the piston, along with the crank-shaft, etc., are the mechanical parts.
(iv) The agents which transfer the energy from the engine to the external agents are mechanical parts. The cylinder with the piston, along with the crank-shaft, etc., are the mechanical parts.
 (iv) The agents which transfer the energy from the engine to the external agents are mechanical parts. The cylinder with the piston, along with the crank-shaft, etc., are the mechanical parts.
(iv) The agents which transfer the energy from the engine to the external agents are mechanical parts. The cylinder with the piston, along with the crank-shaft, etc., are the mechanical parts.
Comments
Post a Comment