Q1. Is it possible that there is no increase in the temperature of a body despite being heated?
Solution
Yes, during the change of state (such as melting of ice, boiling of water, etc.), the system absorbs heat but its temperature does not change. In such a case, only the internal energy changes.
Q2. Which of the three modes of transfer of heat is the fastest?
Solution
Radiation is the fastest mode of heat transfer its velocity being 3 x 108 m/s in vacuum. For example: the heat from the Sun comes to Earth in the form of radiation and it takes approximately 8 minutes for the solar rays to reach Earth.
Q3. The triple point of carbon dioxide is 216.55 K. Express this temperature on Fahrenheit scale. (Take absolute zero as 273 K)
Solution
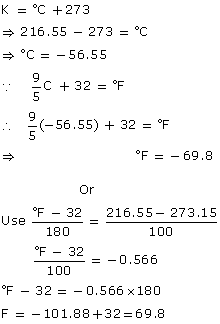
Q4. Answer the following:
The triple-point of water is a standard fixed point in modern thermometry. Why? What is wrong in taking the melting point of ice and the boiling point of water as standard fixed points (as was originally done in the Celsius scale)?
Solution
It is because of the fact that triple point of a substance is unique, i.e. it occurs at one particular set of values of pressure and temperature. The melting point of ice and boiling point of water are not unique. They change with change in value of pressure or due to the presence of impurities in water.
Q5. Water is used in hot water bottles and coolants as well. Why?
Solution
Water is used in hot water bottles and coolants as well because specific heat of every liquid varies with the temperature. But water shows a very peculiar behavior that is at
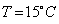 , its specific heat is
, its specific heat is 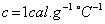
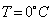 , its specific heat is
, its specific heat is 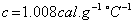
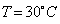 , its specific heat is
, its specific heat is 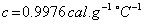 Since the specific heat of water is large, therefore, by absorbing or releasing large amounts of heat, temperature of water varies or changes by small amounts. Hence it is used in hot water bottles and also as coolant in radiators.
Since the specific heat of water is large, therefore, by absorbing or releasing large amounts of heat, temperature of water varies or changes by small amounts. Hence it is used in hot water bottles and also as coolant in radiators.
Q6. What kind of thermometers are used to measure high temperatures like 5000oC ?
Solution
Radiation Thermometers or pyrometers are used to measure high temperatures like 5000oC.
Q7. By what mode of heat transfer is mercury heated?
Solution
Mercury is available in liquid form but it gets heated by conduction and not by convection method as in most of the liquids.
Q8. Assume that the thermal conductivity of copper is four times that of brass. Two rods of copper and brass, of the same length and cross section are joined end to end. The free end of the copper rod is kept it 0 °C and the free end of the brass rod at 100 °C. Calculate the temperature at the junction of the two rods at equilibrium. Ignore radiation losses.
Solution
Let the thermal conductivity of brass be K.
Hence, thermal conductivity of copper = 4 K
Length of each rod = x
Suppose θ is the temperature of the junction of the two rods in equilibrium.
Rate of flow of heat energy through brass = rate of flow of heat energy through copper
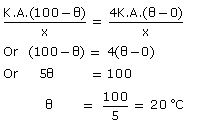
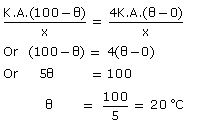
Q9. Explain why:
(i) A body with large reflectivity is a power emitter.
(ii) A brass tumbler feels much colder than a wooden tray on a chilly day.
(iii) An optical pyrometer (for measuring high temperatures) calibrated for an ideal black body radiation gives too low a value for the temperature of a red hot piece in the open, but gives a correct value for the temperature when the same piece is in the furnace.
(iv) The earth without its atmosphere would be inhospitably cold.
(v) Heating systems based on circulation of steam are more efficient in warming a building than those based on circulation of hot water.
Solution
(i) The body having a large reflectivity (or bright surface) is a poor absorber of heat radiations. Poor absorbers are poor radiators. Therefore, a body with a large reflectivity is a poor emitter.
(ii) Brass is a good conductor of heat. When we touch the brass tumbler, heat is quickly transferred from the fingertips to the tumbler. Thus, we feel cold while touching the brass (or any other) tumbler on a chilly day.
(iii) The temperature of the red hot iron in the oven is given by 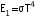 . However, when the iron is taken out in the open (To), then its radiant energy is given by
. However, when the iron is taken out in the open (To), then its radiant energy is given by 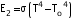 . Therefore, the pyrometer will measure a low value for the red hot iron in the open.
(iv) The atmosphere acts as a blanket over the Earth and does not allow its heat to be radiated during the night.
(v) Because steam contains more heat in the form of latent heat (540 cal/g) than water.
. Therefore, the pyrometer will measure a low value for the red hot iron in the open.
(iv) The atmosphere acts as a blanket over the Earth and does not allow its heat to be radiated during the night.
(v) Because steam contains more heat in the form of latent heat (540 cal/g) than water.
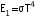 . However, when the iron is taken out in the open (To), then its radiant energy is given by
. However, when the iron is taken out in the open (To), then its radiant energy is given by 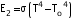 . Therefore, the pyrometer will measure a low value for the red hot iron in the open.
(iv) The atmosphere acts as a blanket over the Earth and does not allow its heat to be radiated during the night.
(v) Because steam contains more heat in the form of latent heat (540 cal/g) than water.
. Therefore, the pyrometer will measure a low value for the red hot iron in the open.
(iv) The atmosphere acts as a blanket over the Earth and does not allow its heat to be radiated during the night.
(v) Because steam contains more heat in the form of latent heat (540 cal/g) than water.
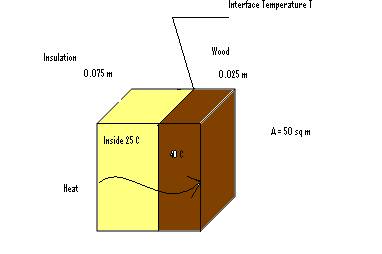
Q10. A wooden plank of 0.025 m is attached to an insulation of 0.075 m. The temperature inside it is 25 ˚C and the outside temperature is 4 ˚C. T is the interface temperature. Take: K(wood)= 0.80 J/s m ˚C, K(insulation)= 0.20 J/s m ˚C. Calculate how much heat energy flows through the wall per second?
Solution

Q11. A 25 cm rod at 12 degree Celsius is heated to 80 degree Celsius. It increases 0.32 cm in length. Find the coefficient of linear expansion of material.
Solution

Q12. Is Cp greater than Cv? Why?
Solution
Yes, Cp is greater than Cv because when a gas is heated at constant volume, the work is done only to increase the internal energy of the system. Whereas, when the gas is heated at constant pressure work is done to overcome this pressure and expand in volume and also to increase the internal energy of the system. Hence, Cp greater than Cv.
Q13. What are the various changes that takes place on heating a substance?
Solution
When we heat a substance it causes:
a. Expansion of the matter
b. Change in its temperature
c. State of the matter changes
d. Chemical properties of the matter changes.
Q14. Explain:
(i) Why does the air pressure in a car tyre during driving increase?
(ii) Why coolant used in a chemical plant should have high specific heat?
Solution
(i) The air pressure in a car tyre during driving increases because work done against friction is converted into heat. Due to which the gas in the trye gets heated, and hence, the pressure of gas increases as P α T at constant volume.
(ii) The coolant used in a chemical plant should have high specific heat because heat absorbed by a substance is directly proportional to specific heat of the substance.
Q15. Does the Fahrenheit scale and Kelvin scale give same reading? If yes at what temperature?
Solution
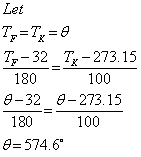
Q16. A solid material is supplied with heat at constant rate. The temperature of the material is changing with the heat input as shown in the figure. Study the graph carefully and answer the following questions.
(i) What do the horizontal regions AB and CD represent?
(ii) If CD= 2AB, what do you infer?
(iii) What does the slope DE represent?
(iv) The slope of OA>the slope of BC. What does this indicate?


Solution
(i) The horizontal portion AB represents the change of state from solid to liquid at constant temperature. This gives the latent heat of fusion. Similarly, CD represents the change of state from liquid to vapour state at constant temperature. This gives the latent heat of vapourisation.
(ii) When CD = 2AB, the latent heat of vapourisation is twice the latent heat of fusion of that substance.
(iii)
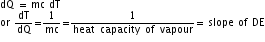 Therefore, the slope of DE represents the reciprocal of heat capacity of the vapour state of the substance.
(iv)
Therefore, the slope of DE represents the reciprocal of heat capacity of the vapour state of the substance.
(iv)
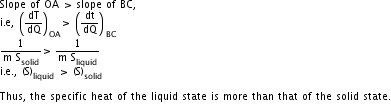
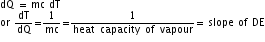 Therefore, the slope of DE represents the reciprocal of heat capacity of the vapour state of the substance.
(iv)
Therefore, the slope of DE represents the reciprocal of heat capacity of the vapour state of the substance.
(iv)
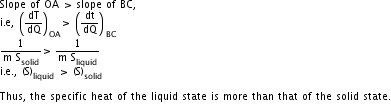
Q17. Which instrument is used to measure temperature? What are the various scales of temperature? Write the expression stating the relation between various scales of temperature.
Solution
Thermometer is used to measure temperature.
The various scales of temperature used are:
a. Celsius scale
b. Fahrenheit scale
c. Kelvin scale
The expression which establishes relation between all the scales is:


Q18. If a liquid is heated from top, then by which mode the transfer of heat would take place?
Solution
If a liquid is heated from top, then the heat transfer would be by conduction process as heated layer of liquid would not move down and so convection could not take place.
Comments
Post a Comment